 |
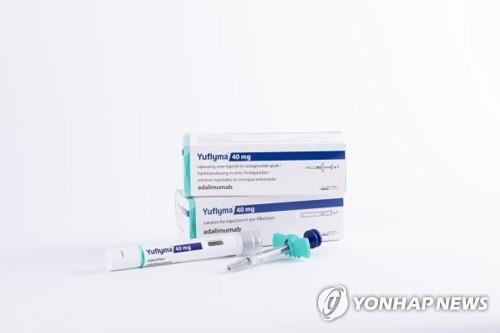
| ▲ This image, provided by South Korean pharmaceutical giant Celltrion Inc., shows its biosimilar Yuflyma. (PHOTO NOT FOR SALE) (Yonhap) |
Celltrion-US sales
Celltrion's autoimmune disease biosimilar to land in U.S. in July 2023
By Kim Han-joo
SEOUL, April 27 (Yonhap) -- South Korean pharmaceutical giant Celltrion Inc. said Wednesday it will begin sales of its autoimmune disease biosimilar Yuflymain in the United States in July 2023.
The plan comes as Celltrion has settled a patent dispute with U.S. drugmaker AbbVie Inc.
Yuflyma -- a biosimilar referencing blockbuster drug Humira -- is used to treat patients with multiple chronic inflammatory diseases, such as rheumatoid arthritis and psoriasis.
Celltrion sought a marketing authorization with the U.S. Food and Drug Administration in November, 2021, with an approval expected within this year.
The high-concentration biosimilar is already being marketed in major European countries and South Korea following its approval by drug authorities.
Yuflyma requires only half the solution administered to patients compared with the existing Humira biosimilar and is also citrate-free, which lessens discomfort during injection, Celltrion said.
(END)
(C) Yonhap News Agency. All Rights Reserved























![[가요소식] TXT](/news/data/20251220/yna1065624915960696_833_h2.jpg)







